Effective Typhoid Test Kit: H. Pylori Ab Rapid Test Strip/Device
PRINCIPLE
The H. Pylori Ab Rapid Test Strip (Serum/ Plasma) is a qualitative membrane strip based immunoassay for the detection of H. Pylori antibodies in whole blood, serum or plasma. In this test procedure, the test strip is immersed in the specimen or specimen containing buffering solution. This specimen migrates chromatographically along the length of the test strip and interacts with the reagents contained on the test strip. If the specimen contains H. Pylori antibodies, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain H.
Pylori antibodies, a colored line will not appear in this region indicating a negative result. To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
Brand: QL
Specimens: : Serum/Plasma
Reading time:20 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Individually packed test devices/strips
Disposable pipettes
Buffer
Package insert
Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time:20 minutes.
Pack:25 T
STORAGE: 2‐30°C
Individually packed test devices/strips
Disposable pipettes
Buffer
Package insert
PROCEDURE
- Allow the test device, specimen, buffer, and/or controls to equilibrate to room temperature (15-30°C) prior to testing.
- Remove the test device from the foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
- Place the test device on a clean and level surface.
- Hold the dropper vertically and transfer 2 drops of sample to the specimen well (S) of the test device, then add 1 drop of buffer and start the timer.
- Wait for the red line(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION OF RESULTS
-
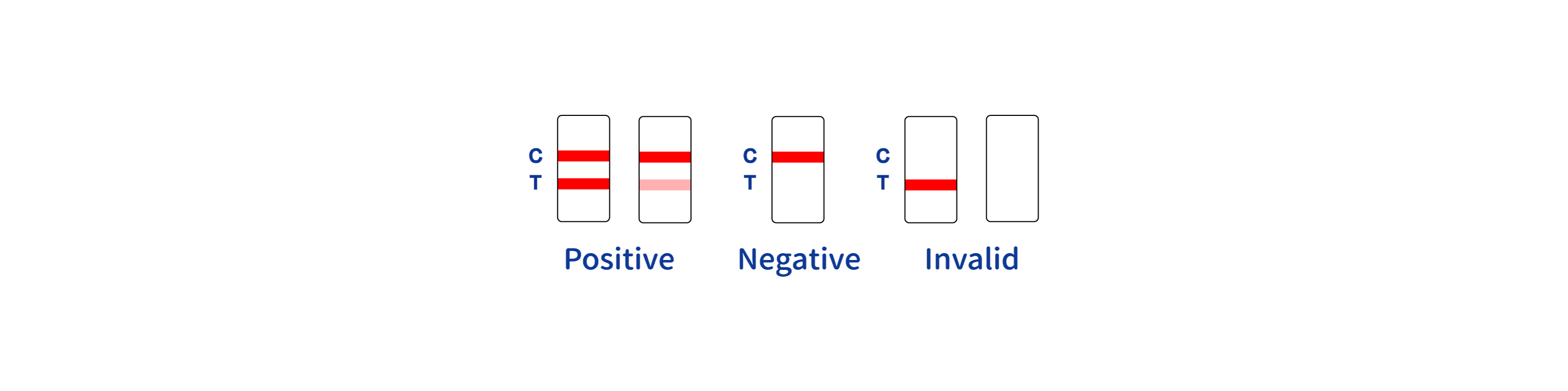
POSITIVE RESULT:
* A colored band appears in the control band region (C) and another colored band appears in the T band region.
NEGATIVE RESULT:
One colored band appears in the control band region (C). No band appears in the test band region (T).
INVALID RESULT:
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
NOTE:
1. The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
At QL Biotech, we strive to create products that set the benchmark for efficiency and innovation. Our Typhoid Test Kit exemplifies this commitment. This rapid test strip/device guarantees quick, accurate results, helping healthcare professionals and individuals to take immediate action for treatment and management of potential health risks. In a nutshell, whether you’re a healthcare professional seeking a reliable tool for clinical diagnostics, or an individual seeking to take control of your health, our Typhoid Test Kit - H. Pylori Ab Rapid Test Strip/Device is an essential tool for you. Trust in QL Biotech for your healthcare needs - because your health is our priority.


