Innovative Rota Test: QL Biotech's Advanced Rotavirus/Adenovirus Diagnostics
PRINCIPLE
The Rotavirus/Adenovirus Combo Rapid Test Device (Feces) has been designed to detect rotavirus and adenovirus through visual interpretation of color development in the internal strip. The membrane was immobilized with anti-rotavirus antibodies and anti-adenovirus on the test region. During the test, the specimen is allowed to react with colored anti rotavirus antibodies colloidal gold conjugates and anti-adnovirus antibodies colloidal gold conjugates, which were precoated on the sample pad of the test. The mixture then moves on the membrane by a capillary action, and interact with reagents on the membrane. If there were enough rotavirus in specimens, a colored band will form at the R region of the membrane. Similarily, If there were enough adenovirus in specimens, a colored band will form at the A region of the membrane. Presence of colored band(s) indicates a positive result, while its absence indicates a negative result. Appearance of a colored band at the control region serves as a procedural control. This indicates that proper volume of specimen has been added and membrane wicking has occurred.
Product Detail
Brand:QL
Specimens: : Feces
Reading time:10 minutes.
Pack:20 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
- Individually packed test devices
- Specimens dilution tube with buffer
- Disposable pipettes
- Package insert
PROCEDURE
- Bring tests, specimens, buffer and/or controls to room temperature (15- 30°C) before use.
1. Specimen collection and pre-treatment:
1) Use the specimen collection container for specimen collection. Best results will be obtained if the assay is performed within 6 hours after collection.
2) For solid specimens: Unscrew and remove the dilution tube applicator. Be careful not to spill or spatter solution from the tube. Collect specimens by inserting the applicator stick into at least 3 different sites of the feces to collect approximately 50 mg of feces (equivalent to 1/4 of a pea).
For liquid specimens: Hold the pipette vertically, aspirate fecal specimens, and then transfer 2 drops (approximately 50 µL) into the specimen collection tube containing the extraction buffer.
3) Place the applicator back into the tube and screw the cap tightly. Be careful not to break the tip of the dilution tube.
4) Shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Specimens prepared in the specimen collection tube may be stored for 6 months at -20°C if not tested within 1 hour after preparation.
2. Testing
1) Remove the test from its sealed pouch, and place it on a clean, level surface.
Label the test with patient or control identification. To obtain a best result, the assay should be performed within one hour.
2) Using a piece of tissue paper, break the tip of the dilution tube. Hold the tube vertically and dispense 3 drops of solution into the specimen well (S) of the test device.
Avoid trapping air bubbles in the specimen well (S), and do not drop any solution in observation window.
As the test begins to work, you will see color move across the membrane.
3. Wait for the colored band(s) to appear. The result should be read at 10 minutes.
Do not interpret the result after 20 minutes.
Note: If the specimen does not migrate (presence of particles), centrifuge the extracted specimens contained in the extraction buffer vial. Collect 80 µL of supernatant, dispense into the specimen well (S) of a new test device and start afresh following the instructions mentioned above.
INTERPRETATION OF RESULTS
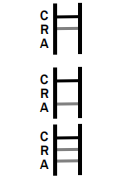
POSITIVE: * Rotavirus Positive: * A colored band appears in the control band region (C) and another colored band appears in the R band region.
Adenovirus Positive: * A colored band appears in the control band region (C) and another colored band appears in the A line region.
Rotavirus and Adenovirus Positive: * A colored band appears in the control line region (C) and two other colored bands appear in A line region and R line region respectively.

NEGATIVE: One colored band appears in the control band region (C). No band appears in the test band region (A/R).
INVALID RESULT:

Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
NOTE:
The intensity of the color in test region (A/R) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.Insufficient specimen volume, incorrect operation procedure, or performingexpired tests are the most likely reasons for control band failure.
Our Rota Test exemplifies the spirit of innovation that drives QL Biotech. Meticulously designed to enhance diagnostic practices, this combined Rotavirus/Adenovirus Rota Test is a vital tool in every medical professional's arsenal. By choosing QL Biotech's Rota Test, you choose reliability, accuracy, and most importantly, you choose a rapid response to the diverse challenges presented by rotavirus and adenovirus infections. At QL Biotech, we merge sophisticated technology with convenience, ensuring our products are not only efficient but also user-friendly. Our Rota Test Device signifies this promise and more, offering an innovative approach to the rapid detection of rotavirus and adenovirus. Commit to QL Biotech's Rota Test today, and embrace the future of advanced diagnostics.


