Innovative Tuberculosis Diagnosis Test by QL Biotech
PRINCIPLE
The H. Pylori Ag Rapid Test Devic/Strip (Feces) is a non‐invasive lateral flow assay, rapid, precise and easy to perform.This test makes use of specific antibodies against H. Pylori antigen adsorbed onto a reactive membrane. If H. Pylori is present in stool specimen, the specific antigen is bound by the second antibody which is conjugated with colloidal gold particles. A generic antibody, fixed onto the reactive membrane, in shape of the band, is able to capture the second conjugated antibody, assuring the correctness of the test performance.
Product Detail
Brand: QL
Specimens: : Feces
Reading time:10 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Individually packed Test Devices/Strips
Each test contains a strip with colored conjugates and reactive reagents pre-spreaded at the
corresponding regions
Tubes with buffer
Phosphate buffered saline and preservative, extract the samples
Package insert
For operation instruction
PROCEDURE
- Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
1. Specimen collection and pre-treatment:
1) Unscrew and remove the dilution tube applicator. Be careful not to spill or spatter solution from the tube. Collect specimens by inserting the applicator stick into at least 3 different sites of the feces.
2) Place the applicator back into the tube and screw the cap tightly. Be careful not to break the tip of the dilution tube.
3) Shake the specimen collection tube vigorously to mix the specimen and the extraction buffer. Specimens prepared in the specimen collection tube may be stored for 6 months at -20°C if
not tested within 1 hour after preparation.
2. Testing
1) Remove the test from its sealed pouch, and place it on a clean, level surface. Label the test with patient or control identification. To obtain a best result, the assay should be performed within one hour.
2) Using a piece of tissue paper, remove the tip of the dilution tube. Hold the tube vertically and dispense 3 drops of solution into the sample well of the Test Device.
Avoid trapping air bubbles onto the sample pad, and do not drop any solution in observation window.
As the test begins to work, you will see color move across the membrane.
3. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION OF RESULTS
-
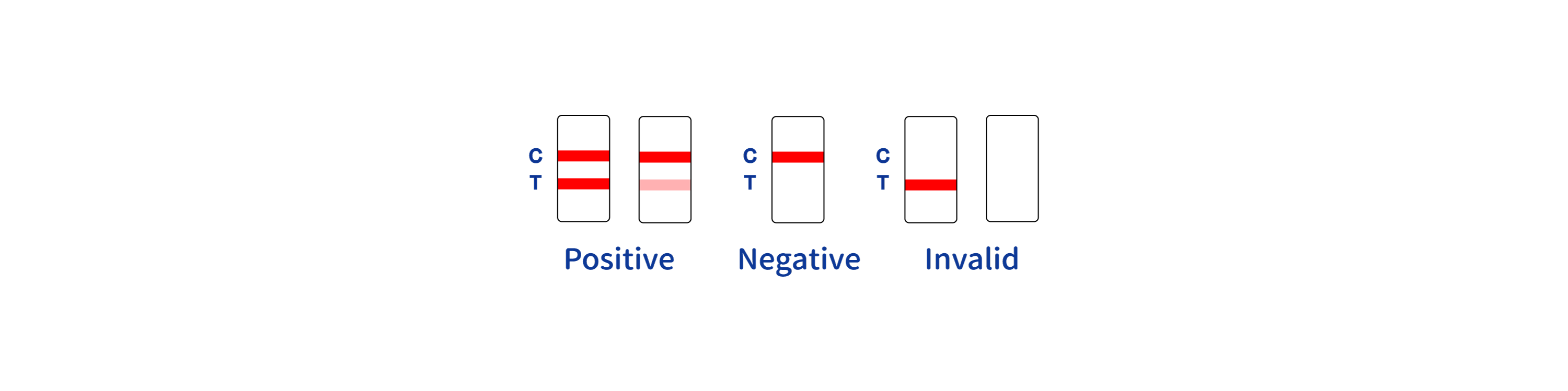
POSITIVE RESULT:
A colored band appears in the control band region (C) and another colored band appears in the T band region
NEGATIVE RESULT:
One colored band appears in the control band region (C). No band appears in the test band region (T)
INVALID RESULT:
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
NOTE:
1. The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
At QL Biotech, we are committed to enhancing the capabilities of medical professionals in the fight against TB. Our dedication to research and development in biotechnological solutions is reflected in the superior design and functionality of the H.Pylori Ag Rapid Test Device/Strip. By choosing our product, healthcare providers can ensure that they are equipped with the latest in TB diagnostic technology, enabling them to make informed decisions quickly and accurately. In conclusion, as TB continues to pose a significant global health challenge, the need for innovative diagnostic solutions has never been more critical. QL Biotech's H.Pylori Ag Rapid Test Device/Strip represents a significant leap forward in meeting this need, providing a rapid, accurate, and user-friendly tuberculosis diagnosis test that is unparalleled in its reliability and effectiveness. Join us in our mission to combat tuberculosis with the best tools science and technology have to offer.


