QL Biotech sFlT-1/PIGF Test: iGFBP-1 Rapid Test Device & Strip
Product Parameters
|
Product Name |
iGFBP-1 Rapid Test Device |
iGFBP-1 Rapid Test Strip |
|
Used for |
For professional in vitro diagnostic use only. |
|
|
Specimen |
Vaginal secretion |
|
|
Packing |
20 tests/box , 1 test/polybag |
|
|
MOQ |
1000 tests |
|
|
Diverse Coopetation Modes |
OEM/ODM |
|
STORAGE AND STABILITY
● The kit should be stored at 2‐30°C until the expiry date printed on the sealed pouch.
● The test must remain in the sealed pouch until use.
● Do not freeze.
● Cares should be taken to protect components in this kit from contamination. Do not use if there is evidence of microbial contamination or precipitation. Biological contamination of dispensing equipments, containers or reagents can lead to false results.
INTERPRETATION OF RESULTS
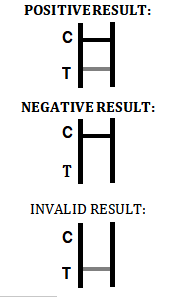
POSITIVE RESULT:
*A colored band appears in the control band region (C) and another colored band appears in the T band region.
NEGATIVE RESULT:
One colored band appears in the control band region (C). No band appears in the test band region (T).
INVALID RESULT:
Control band fails to appear. Results from any test which has not
produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
NOTE:
1.The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
2.Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
1.The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
2.Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Experience the QL Biotech difference with our iGFBP-1 Rapid Test Device and Strip. Incorporate these vital tools into your diagnostic processes, elevating their accuracy and reliability, and ensure the delivery of the best healthcare services. Being a part of the renowned sFlT-1/PIGF Test range, these devices and strips guarantee results that you can trust, thereby enhancing patient trust and satisfaction. Investing in our iGFBP-1 Rapid Test Device and Strip equals investing in quality, reliability, and concentrated industry expertise. Trust QL Biotech's sFlT-1/PIGF Test range for all your in vitro diagnostic needs and experience an upgrade in your diagnostic capabilities. Partner with us, and let’s work together towards delivering superior healthcare.


