Rapid Dengue IgG/IgM Test - Detect Toxoplasma Gondii Effectively
Product Detail
Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time: in 15 minutes.
Pack:25 T
STORAGE 2‐30°C
KIT COMPONENTS
● Individually packed test devices
Each device contains a strip with colored conjugates and reactive reagents pre-spreaded at the corresponding regions
● Disposable pipettes
For adding specimens use
● Buffer
Phosphate buffered saline and preservative
● Package insert
For operation instruction
1.Dengue IgG/IgM Rapid Test Device Package Insert
PRINCIPLE
The Dengue IgG/IgM Rapid Test Device (Whole Blood/Serum/Plasma) is a qualitative membrane‐based immunoassay for the detection of Dengue antibodies in whole blood, serum, or plasma. This test consists of two components, an IgG component and an IgM component. In the Test region, anti‐human IgM and IgG is coated.
During testing, the specimen reacts with Dengue antigen‐coated particles in the test strip. The mixture then migrates upward on the membrane chromatographically by capillary action and reacts with the anti‐human IgM or IgG in test line region. If the specimen contains IgM or IgG antibodies to Dengue, a colored line will appear in test line region.
Therefore, if the specimen contains Dengue IgM antibodies, a colored line will appear in test line region 1. If the specimen contains Dengue IgG antibodies, a colored line will appear in test line region 2. If the specimen does not contain Dengue antibodies, no colored line will appear in either of the test line regions, indicating a negative result. To serve as a procedural control, a colored line will always appeared in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
ASSAY PROCEDURE
Bring the specimen and test components to room temperature Mix the specimen well prior to assay once thawed. Place the test device on a clean, flat surface.
Forwholebloodsample:
Fill the dropper with the specimen then add 1 dropper of specimen into the sample well. The volume is around 10µL. Making sure that there are no air bubbles. Then add 2 drops (about 80 µL) of Sample Diluent immediately into the sample well.
For Plasma/ Serum sample:
Fill the dropper with the specimen not to exceed the specimen line. The volume of the specimen is around 5µL.
Dispense the entire specimen into the center of the sample well making sure that there are no air bubbles. Then add 2 drops (about 80 µL) of Sample Diluent immediately into the sample well.
Note: Practice a few times prior to testing if you are not familiar with the mini dropper. For better precision,transfer specimenbyapipettecapabletodeliver5µLofvolume.
Set up a timer. Read the result at 15 minutes. Don’t readresultafter30minutes.Toavoidconfusion,discard thetestdeviceafter interpretingtheresult.
2.Dengue NS1 Rapid Test Device Package Insert
The Dengue NS1 Rapid Test Device is a lateral flow chromatographic immunoassay for the qualitative detection of dengue virus antigen (Dengue Ag) in human whole blood, serum or
plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with Dengue viruses. Any reactive specimen with the Dengue NS1 Rapid Test Device must be confirmed with alternative testing method(s) and clinical findings
ASSAY PROCEDURE
Step 1: Bring the specimen and test components to room temperature if refrigerated or frozen. Mix the specimen well prior to assay once thawed.
Step 2: When ready to test, open the pouch at the notch and remove device. Place the test device on a clean, flat surface.
Step 3: Be sure to label the device with specimen’s ID number.
Step 4: For whole blood samples:
Fill the dropper with the specimen then add 2 drops (about 80µL) of specimen and 2 drops of buffer into sample well, making sure that there are no air bubbles.
For Plasma/ Serum samples:
Fill the plastic dropper with the specimen. Holding the dropper vertically, dispense 1 drop (about 40µL) of specimen and 2 drops of buffer into the sample well,making sure that there are no air bubbles.
Step 5: Set up a timer.
Step 6: Read the result at 15 minutes.
Don’t read results after 30 minutes. To avoid confusion, discard the test device after
interpreting the result.
3.Dengue IgG/IgM/NS1 Combo Rapid Test Device Package Insert
The Dengue IgG/IgM/NS1 Combo Rapid Test Device is a lateral flow chromatographic immunoassay for the qualitative detection of dengue IgG/ IgM and virus antigen (Dengue Ag) in human whole blood, serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with Dengue viruses. Any reactive specimen with the Dengue IgG/IgM/NS1 Combo Rapid Test Device must be confirmed with alternative testing method(s) and clinical findings.
ASSAY PROCEDURE
Step 1: Bring the specimen and test components to room temperature if refrigerated or frozen. Mix the specimen well prior to assay once thawed.
Step 2: When ready to test, open the pouch at the notch and remove device. Place the test device on a clean, flat surface.
Step 3: Be sure to label the device with specimen’s ID number.
Step 4: For whole blood samples:
Fill the dropper with the specimen then add 1 drop (about 10µL) of specimen and 2 drops of buffer into the IgG/ IgM sample well and 4 drops of specimen and 2 drops of buffer into NS1 sample well, making sure that there are no air bubbles.
For Plasma/ Serum samples:
Fill the plastic dropper with the specimen. Holding the dropper vertically, dispense 5 µL of specimen and 2 drops of buffer into the IgG/ IgM sample well and 4 drops of specimen and 1 drops of buffer into the NS1 sample well, making sure that there are no air bubbles.
Step 5:Set up a timer.
Step 6: Results can be read in 15 minutes. Positive results can be visible in as short as 1 minute.
Don’t read results after 30 minutes. To avoid confusion, discard the test device after
interpreting the result
INTERPRETATION OF ASSAY RESULT
Dengue NS1
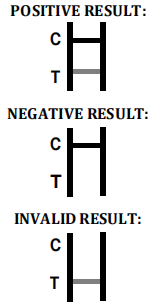
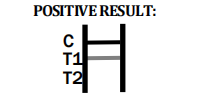
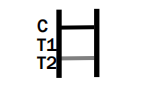
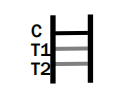
Dengue IgG/IgM
IgG Positive
IgM Positive
IgG and IgM Positive
NEGATIVE RESULT
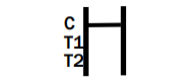
INVALID RESULT
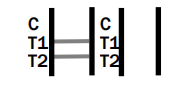
Dengue IgG/IgM/NS1
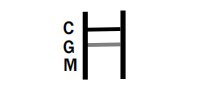
POSITIVE RESULT:
IgG POSITIVE:
IgM POSITIVE:
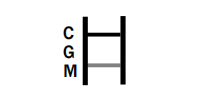
IgG AND IgM POSITIVE: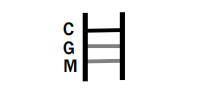
Understanding the growing concern and need for effective diagnostic methods for Toxoplasma Gondii, a parasite that poses significant health risks, especially to pregnant women and individuals with compromised immune systems, our product also comes into play. Although primarily designed for Dengue detection, the meticulous design and technology behind the Dengue IgG/IgM Rapid Test Device have positioned it as a potential tool in the broader spectrum of parasitic infection diagnosis, including Toxoplasma Gondii. Through ongoing research and development, QL Biotech is committed to enhancing the capabilities of our products to address a wider array of infectious diseases, ultimately contributing to better health outcomes globally. With QL Biotech's Dengue IgG/IgM Rapid Test Device, healthcare providers now have access to a fast, efficient, and reliable testing method. This breakthrough device not only signifies a leap forward in the fight against Dengue fever but also highlights our commitment to expanding our diagnostic capabilities to include critical concerns such as Toxoplasma Gondii infections.




