Simplified TOXO IgG/IgM Detection - Torch Rapid Test Kit
PRINCIPLE
The TB Antibody Rapid Test device is a lateral flow chromatographic immunoassay based on the principle of the double antigen–sandwich technique. The test cassette consists of: 1) a burgundy colored conjugate pad containing M.TB antigens conjugated with colloid gold (M.TB conjugates)and rabbit IgG‐gold conjugates, 2) a nitrocellulose membrane strip containing a test band (T band) and a control band (C band). The T band is pre‐coated with non‐conjugated M.TB antigens, and the C band is pre‐coated with goat anti‐rabbit IgG. When an adequate volume of test specimen is dispensed into the sample well of the cassette, the specimen migrates by capillary action across the cassette. The antibodies: either the IgG, the IgM to M. TB if present in the specimen will bind to the M.TB conjugates. The immunocomplex is then captured on the membrane by the pre‐coated M.TB antigens, forming a burgundy colored T band, indicating a M.TB Ab positive test result. Absence of the T band suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti‐rabbit IgG/rabbit IgG‐gold conjugate regardless the presence of any antibodies to M.TB. Otherwise, the test result is invalid and the specimen must be retested with another device.
Product Detail
-
-
-
-
-
-
1.Brand: QL
Specimens: : Serum/Plasma
Reading time:15 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS(Device)
Individually packed test devices
Disposable pipettes
Buffer
Package insert
2.Brand: QL
Specimens: : Whole Blood/Serum/Plasma
Reading time:15 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS
Individually packed test devices
Disposable pipettes
Buffer
Package insert
-
-
-
-
-
-
PRINCIPLE
-
It is a qualitative membrane strip based immunoassay for the detection of TOXO antibodies (IgG and IgM) in Whole Blood /Serum / Plasma. The test device consists of: 1) a burgundy colored conjugate pad containing TOXO recombinant envelope antigens conjugated with Colloid gold (TOXO conjugates) and rabbit IgG-gold conjugates,2) a nitrocellulose membrane strip containing two test bands (T1 and T2 bands) and a control band (C band). The T1 band is pre-coated with the antibody for the detection of IgM anti-TOXO, T2 band is coated with antibody for the detection of IgG anti-TOXO, and the C band is pre-coated with goat anti rabbit IgG. When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. IgG anti-TOXO, if present in the specimen, will bind to the TOXO conjugates. The immunocomplex is then captured by the reagent pre-coated on the T2 band, forming a burgundy colored T2 band, indicating a TOXO IgG positive test result and suggesting a recent or repeat infection. IgM anti-TOXO if present in the specimen will bind to the TOXO conjugates. The immunocomplex is then captured by the reagent coated on the T1 band, forming a burgundy colored T1 band, indicating a TOXO IgM positive test result and suggesting a fresh infection. Absence of any T bands (T1 and T2) suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti rabbit IgG/rabbit IgG-gold conjugate regardless of the color development on any of the T bands. Otherwise, the test result is invalid and the specimen must be retested with another device.
TEST PROCEDURE
Allow the test, specimen, buffer and/or controls to reach room temperature 15-30℃ (59-86℉) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and level surface.
3. Hold the dropper vertically and transfer 1 drop of specimen (approximately 10μl) to the specimen well(S) of the test device, then add 2 drops of buffer (approximately 80μl) and start the timer. See illustration below.
4. Wait for the colored line(s) to appear. Read results at 15 minutes. Do not interpret the result after 20 minutes.
Notes:
Applying sufficient amount of specimen is essential for a valid test result. If migration (the wetting of membrane) is not observed in the test window after one minute, add one more drop of buffer to the specimen well. -
INTERPRETATION OF RESULTS
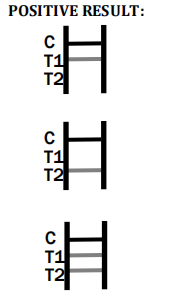
IgM Positive:* The colored line in the control line region (C) appears and a colored line appears in test line region 1 (T1). The result is positive for Toxo virus specific-IgM antibodies and is indicative of primary Toxo infection.
IgG Positive:* The colored line in the control line region (C) appears and a colored line appears in test line region 2 (T2). The result is positive for Toxo virus specific-IgG and is probably indicative of secondary Toxo infection.
IgG and IgM Positive:* The colored line in the control line region (C) appears and two colored lines should appear in test line regions 1 and 2 (T1 and T2). The color intensities of the lines do not have to match.
The result is positive for IgG & IgM antibodies and is indicative of secondary Toxo infection.
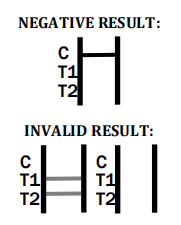
The colored line in the control line region (C) appears. No line appears in test line regions 1 or 2 (T1 or T2).
INVALID RESULT:
Control line (C) falls to appear. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the procedure with a new test devic immediately and contact your local distributor.
NOTE:
1.The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level cannot be determined by this qualitative test.2.Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Embarking on this diagnostic voyage, users will appreciate the test’s streamlined process, which has been developed with the end-user in mind. From the collection of the sample to the interpretation of results, every step is simplified to ensure clarity and efficiency. The test’s intuitive design is complemented by its rapid turnaround time, delivering results in just minutes. This efficiency does not come at the expense of accuracy, as the One Step TOXO IgG/IgM Test kit is meticulously calibrated to provide results that healthcare professionals can trust. As the landscape of medical diagnostics evolves, QL Biotech remains at the forefront, embracing innovation and quality. Our Torch Rapid Test kit is more than just a product; it is a testament to our dedication to advancing healthcare and improving patient outcomes. Whether in a hospital, clinic, or field setting, the One Step TOXO IgG/IgM Test kit stands ready to meet the challenges of today's healthcare demands, offering a seamless blend of reliability, efficiency, and ease of use.


