T-Ferritin Strip: Rapid PSA Test Device for Whole Blood/Serum/Plasma
SUMMARY
Prostate specific antigen (PSA) is produced by prostate glandular and endothelial cells. It is a single chain glycoprotein with a molecular weight of approximately 34 kDa.1 PSA exists in three major forms circulating in the serum. These forms are free PSA, PSA bound to α1–Antichymotrypsin (PSA‐ACT) and PSA complexed with α2–macroglobulin (PSA‐MG).2 PSA has been detected in various tissues of the male urogenital system but only prostate glandular cells and endothelial cells secrete it. The PSA level in the serum of healthy men is between 0.1 ng/mL and 2.6 ng/mL. It can be elevated in malignant conditions such as prostate cancer, and in benign conditions such as benign prostatic hyperplasia and prostatitis. A PSA level of 4 to 10 ng/mL is considered to be in the “gray‐zone” and above 10 ng/mL is highly indicative of cancer.3 Patients with PSA values between 4‐10 ng/mL should undergo further analysis of the prostate by biopsy. Authorities have begun to explore the possibility of using PSA levels lower than 4.0 ng/mL as the upper limit of normal for screening examinations. By lowering the PSA cutoff from 4 to 3 ng/mL, an increase in cancer detection by 13.2% can be achieved.4 The prostate specific antigen test is the most valuable tool available for the diagnosis of early prostate cancer. Many studies have confirmed that the presence of PSA is the most useful and meaningful tumor marker known for prostate cancer and prostate infection of Benign Prostatic Hyperplasia (BPH).5 The PSA Ultra Prostate Specific Antigen Rapid Test Strip (Whole Blood/Serum/Plasma) utilizes a combination of colloidal gold conjugate and anti‐PSA antibodies to selectively detect all three forms of PSA in whole blood, serum or plasma. The test has a cut‐off value of 4 ng/mL.
Product Parameters
|
Product Name |
Prostate Specific Antigen Rapid Test Device |
Prostate Specific Antigen Rapid Test Strip |
|
Used for |
For professional in vitro diagnostic use only. |
|
|
Specimen |
Whole Blood/Serum/Plasma |
|
|
Packing |
25 tests/box , 1 test/polybag |
|
|
MOQ |
1000 tests |
|
|
Diverse Coopetation Modes |
OEM/ODM |
|
INTERPRETATION OF RESULTS
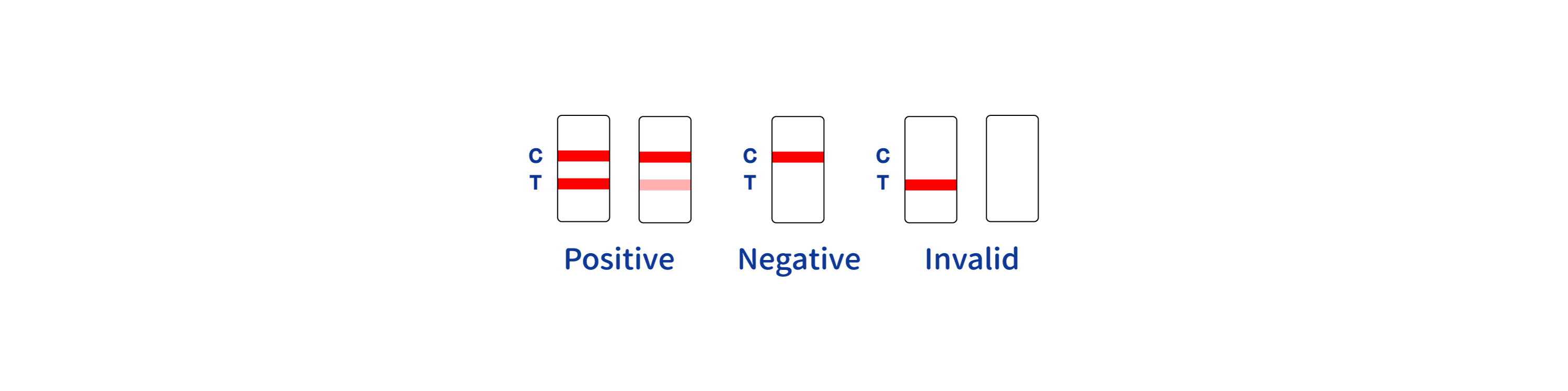
POSITIVE RESULT: A colored band appears in the control band region (C) and another colored band appears in the T band region.
NEGATIVE RESULT: One colored band appears in the control band region (C). No band appears in the test band region (T).
INVALID RESULT:Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.
INTERPRETATION OF RESULTS
Sensitivity and Specificity
The PSA Prostate Specific Antigen Rapid Test Device (Whole Blood/Serum/Plasma) has been tested with a leading commercial PSA EIA test using clinical specimens.
|
Method |
EIA |
Total Results |
||
|
Ultra PSA Rapid Test Device |
Results |
Positive |
Negative |
|
|
Positive |
154 |
4 |
158 |
|
|
Negative |
2 |
266 |
268 |
|
|
Total Results |
156 |
270 |
426 |
|
Relative Sensitivity: 98.7% (97%‐100%)*
Relative Specificity: 98.5% (97.1%‐99.9%)*
Accuracy: 98.6% (97.5%‐99.7%)* *95% Confidence Interval
The T-Ferritin Strip represents a significant milestone in diagnostic technology, setting a new standard for rapid testing devices. With its ease of use and unparalleled accuracy, it has the potential to transform how PSA tests are conducted, influencing healthcare trends positively. Embrace the future of healthcare with the T-Ferritin Strip by QL Biotech, a truly transformative solution to PSA testing, and experience the difference that innovation can bring to diagnosing and managing prostate health.


