Tuberculosis Rapid Test Device - Strep A IVDR Compliant
PRINCIPLE
The TB Antibody Rapid Test device is a lateral flow chromatographic immunoassay based on the principle of the double antigen–sandwich technique. The test cassette consists of: 1) a burgundy colored conjugate pad containing M.TB antigens conjugated with colloid gold (M.TB conjugates)and rabbit IgG‐gold conjugates, 2) a nitrocellulose membrane strip containing a test band (T band) and a control band (C band). The T band is pre‐coated with non‐conjugated M.TB antigens, and the C band is pre‐coated with goat anti‐rabbit IgG. When an adequate volume of test specimen is dispensed into the sample well of the cassette, the specimen migrates by capillary action across the cassette. The antibodies: either the IgG, the IgM to M. TB if present in the specimen will bind to the M.TB conjugates. The immunocomplex is then captured on the membrane by the pre‐coated M.TB antigens, forming a burgundy colored T band, indicating a M.TB Ab positive test result. Absence of the T band suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti‐rabbit IgG/rabbit IgG‐gold conjugate regardless the presence of any antibodies to M.TB. Otherwise, the test result is invalid and the specimen must be retested with another device.
Product Detail
-
-
-
-
-
Brand: QL
Specimens: :Plasma/ Serum/ Whole Blood
Reading time:1 minutes.
Pack:25 T
STORAGE: 2‐30°C
KIT COMPONENTS
- Test devices
- Droppers
- Buffer
- Package insert
-
-
-
-
-
TEST PRINCIPLE
-
The TB IgG/IgM Rapid Test Device (Whole Blood/Serum/Plasma) is a lateral flow chromatographic immunoassay based on the principle of the double antigen–sandwich technique. The test cassette consists of: 1) a burgundy colored conjugate pad containing M.TB antigens conjugated with colloid gold (M.TB conjugates) and rabbit IgG‐gold conjugates, 2) a nitrocellulose membrane strip containing a test band (T band) and a control band (C band). The T band is pre‐coated with non‐conjugated M.TB antigens, and the C band is pre‐coated with goat anti‐rabbit IgG.
When an adequate volume of test specimen is dispensed into the sample well of the cassette, the specimen migrates by capillary action across the cassette. The antibodies: either the IgG, the IgM to M. TB if present in the specimen will bind to the M.TB conjugates. The immunocomplex is then captured on the membrane by the pre‐coated M.TB antigens, forming a burgundy colored T band, indicating a M.TB Ab positive test result.
Absence of the T band suggests a negative result. The test contains an internal control (C band) which should exhibit a burgundy colored band of the immunocomplex of goat anti‐rabbit IgG/rabbit IgG‐gold conjugate regardless the presence of any antibodies to M.TB. Otherwise, the test result is invalid and the specimen must be retested with another device.
ASSAY PROCEDURE
1. Bring the specimen and test components to room temperature. Place the test device on a clean, flat surface.
2. Be sure to label the device with specimen’s ID number.
3. Fill the plastic dropper with the specimen. Holding the dropper vertically, dispense 2 drops (about 80 µL) of specimen into the sample well making sure that there are no air bubbles. Then add 1 drop of buffer into the sample well. Set up timer.
4. Results can be read in 10 minutes. Positive results can be visible in as short as 1 minute. Don’t read result after 10 minutes. To avoid confusion, discard the test device after interpreting the result. -
-
INTERPRETATION OF ASSAY RESULT
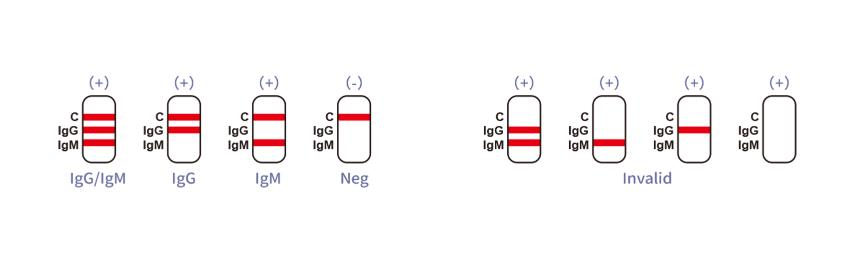
IgG Positive:* The colored line in the control line region (C) appears and a colored line appears in test line region IgG. The result is positive for Tuberculosis specific-IgG antibodies and is indicative of secondary Tuberculosis infection.
IgM Positive:* The colored line in the control line region (C) appears and a colored line appears in test line region IgM. The result is positive for Tuberculosis specific-IgM and is probably indicative of primary Tuberculosis infection.
IgG and IgM Positive:* The colored line in the control line region (C) appears and two colored lines should appear in test line regions IgG and IgM. The color intensities of the lines do not have to match. The result is positive for IgG & IgM antibodies and is indicative of secondary Tuberculosis infection.
*NOTE: The intensity of the color in the test line region(s) will vary depending on the concentration of Tuberculosis antibodies in the specimen. Therefore, any shade of color in the test line region(s) should be considered positive.
NEGATIVE RESULT:
The colored line in the control line region (C) appears. No line appears in test line regions IgG or IgM.
INVALID RESULT:
Control line (C) falls to appear. Insufficient buffer volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the procedure with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
The device's ease of use, combined with its compliance with Strep A IVDR standards, underscores our dedication to offering dependable, user-friendly solutions to the healthcare community. Whether utilized in clinical settings or remote areas with limited access to lab facilities, the Tuberculosis Rapid Test Device stands as a beacon of innovation. It empowers healthcare professionals with the ability to make swift, informed decisions regarding tuberculosis treatment and management, ultimately contributing to improved patient outcomes and a decrease in the spread of this debilitating disease. (QL Biotech's commitment to excellence is evident in every aspect of the Tuberculosis IgG/IgM Rapid Test Device, from its rigorous adherence to Strep A IVDR regulations to its role in advancing global health initiatives. By choosing our product, you are not only selecting a tool of unmatched reliability and accuracy but also supporting a brighter, healthier future for all.)


