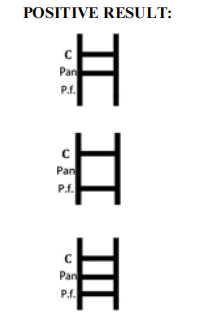
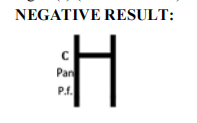
Wholesale Malaria Pf Test Kit for Rapid Diagnostics
Product Main Parameters
| Brand | QL Biotech |
|---|---|
| Specimen | Whole Blood |
| Reading Time | 10 minutes |
| Pack | 25 Tests |
| Storage | 2‐30°C |
| Kit Components | Test devices, Droppers, Buffer, Package insert |
Common Product Specifications
| Test Type | Immuno-chromatographic lateral flow |
|---|---|
| Antigen Targeted | Histidine-rich protein 2 (HRP2) |
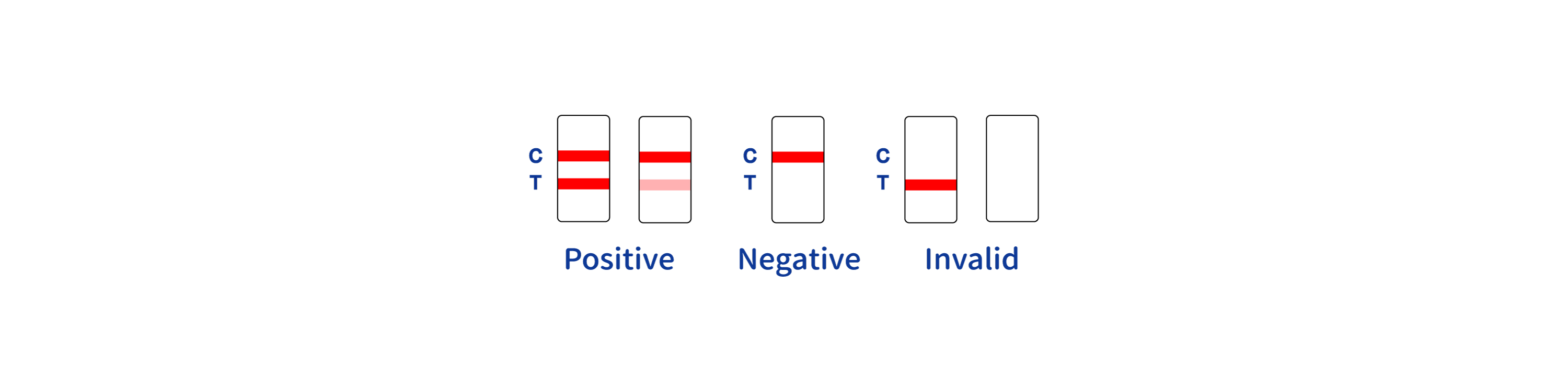
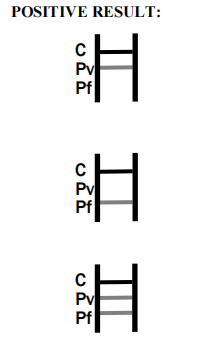
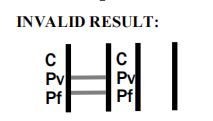
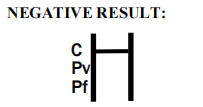
| Result Interpretation | Colored lines on strip |
Product Manufacturing Process
Malaria Pf Test Kits are produced through a precise biotechnological process involving the extraction and stabilization of HRP2 antibodies onto test strips. The manufacturing adheres to stringent quality control standards to ensure the sensitivity and specificity of the diagnostic tests. Continuous improvements in the formulation and production process, as documented in several peer-reviewed studies, have resulted in enhanced detection capabilities. The conclusion from recent authoritative papers suggests that innovations in immunoassay techniques continue to strengthen the reliability of these rapid diagnostic tests, making them indispensable tools in malaria management programs worldwide.
Product Application Scenarios
The wholesale Malaria Pf Test Kit is pivotal in several application scenarios, particularly in remote, resource-limited regions where malaria is endemic. Field researchers and community health workers utilize these kits for onsite testing, enabling immediate diagnosis and treatment decisions. In healthcare facilities, these kits allow for efficient screening and monitoring of malaria cases, contributing to epidemic surveillance and control. As highlighted in authoritative publications, the integration of rapid test kits into national health guidelines has significantly reduced the incidence of untreated malaria and improved healthcare outcomes in affected areas.
Product After-Sales Service
QL Biotech offers comprehensive after-sales support for our wholesale Malaria Pf Test Kits, including customer service for technical inquiries, and assistance with product usage. Warranty and replacement services are also available for defective kits to ensure customer satisfaction and reliability in the field.
Product Transportation
Our Malaria Pf Test Kits are packaged securely to withstand transportation challenges. Shipping is done with temperature-controlled logistics to maintain product integrity, ensuring that the kits arrive in optimal condition for immediate use.
Product Advantages
- Rapid results within 10 minutes.
- Easily interpretable even by minimally trained personnel.
- Compact and transportable, ideal for field use.
- Cost-effective for large-scale deployments.
- Validated by authoritative health organizations for reliability.
Product FAQ
- How accurate is the Malaria Pf Test Kit?
Our wholesale Malaria Pf Test Kit is designed to provide rapid results with high sensitivity and specificity. The kit's accuracy is continually validated through rigorous testing protocols aligned with global health standards, ensuring reliable diagnostic outcomes in varied environmental conditions.
- What is the shelf life of the test kit?
Each test kit comes with a shelf life of 18 months when stored under the recommended temperature conditions of 2‐30°C. Proper storage ensures the kit retains its diagnostic efficacy over the specified period.
- Can the test kit be used for other Plasmodium species?
The primary focus of the Malaria Pf Test Kit is the detection of Plasmodium falciparum. While it is optimized for P.f., other variants of the kit, such as the Malaria Pf/Pan Test Kit, can detect multiple species.
- What are the storage requirements for the test kit?
To ensure the kit's optimal performance, it should be stored in a cool, dry environment away from direct sunlight, maintaining a stable temperature range of 2‐30°C as recommended.
- Is any special training required to use the kit?
No extensive medical training is required to use the Malaria Pf Test Kit. The straightforward instructions included make it accessible for use by healthcare workers with minimal training, making it ideal for remote and resource-limited settings.
- How should discrepancies in test results be addressed?
If discrepancies in test results are encountered, it is advisable to cross-verify with additional diagnostic methods such as microscopy, especially in critical cases. Contacting our technical support team can provide further assistance in such instances.
- Are bulk purchase options available?
Yes, QL Biotech offers competitive wholesale options for bulk purchases of our Malaria Pf Test Kits. Large-scale procurement is facilitated by tailored pricing and logistics support to meet diverse organizational needs.
- What if a kit component is missing or damaged upon delivery?
In the rare event of missing or damaged components, please contact our customer support immediately. We have a responsive replacement policy to address such issues efficiently and maintain service satisfaction.
- What is the procedure for disposal of used test kits?
Used test kits should be disposed of following the local biohazard waste management and disposal regulations to prevent any environmental contamination or health hazards.
- How frequently are new batches of test kits manufactured?
Production is carried out in regular cycles to ensure a continuous supply of fresh kits, aligned with demand forecasts and planned distribution, ensuring timely availability for our wholesale clients.
Product Hot Topics
- Innovations in Malaria Rapid Testing
The advent of advanced rapid test kits like our wholesale Malaria Pf Test Kit has revolutionized disease management, especially in underdeveloped regions. These kits have significantly contributed to reducing malaria-related mortality rates by facilitating timely and accurate diagnosis, thus allowing for prompt treatment and improved patient outcomes.
- Overcoming HRP2 Gene Deletion Challenges
Recent studies have highlighted the challenges posed by HRP2 gene deletions affecting the performance of traditional Malaria Pf Test Kits. The development of alternative biomarkers and enhanced diagnostic strategies are becoming hot topics in the research community, aiming to maintain the kits' efficacy amidst evolving parasite genetics.
- Role of RDTs in Global Malaria Eradication Initiatives
The role of rapid diagnostic tests (RDTs), including our wholesale Malaria Pf Test Kit, is pivotal in the global malaria eradication initiatives orchestrated by the World Health Organization. Their deployment in endemic regions assists in precise data gathering essential for strategic health interventions.
- The Economic Impact of Malaria Testing
Investments in malaria testing infrastructure, such as procuring our wholesale Malaria Pf Test Kit, have far-reaching economic impacts. They reduce the burden on healthcare systems by preventing severe malaria cases, thereby lowering treatment costs and improving workforce productivity.
- Community Health Workers and RDT Implementation
The empowerment of community health workers through the use of accessible diagnostic tools like the wholesale Malaria Pf Test Kit is transforming healthcare delivery. These workers act as frontline defenders in remote areas, ensuring accurate disease surveillance and response.
- Future of Malaria Diagnosis and Treatment
As the landscape of malaria diagnosis advances, the integration of rapid tests like the Malaria Pf Test Kit is anticipated to become more sophisticated with the incorporation of digital health technologies, improving diagnostic accuracy and disease mapping.
- The Importance of Quality Control in RDT Production
Maintaining stringent quality control during the production of rapid diagnostic tests is crucial for reliable performance. QL Biotech's adherence to international standards ensures that our wholesale Malaria Pf Test Kits consistently meet the high demands of clinical diagnostics.
- Training and Education for Effective RDT Use
Effective use of rapid diagnostic tests necessitates comprehensive training programs. Our efforts to provide resources for the correct use of our wholesale Malaria Pf Test Kit highlight the importance of equipping users with the necessary skills for accurate testing.
- Challenges in Malaria Control Amidst Climate Change
Climate change poses significant challenges for malaria control due to shifting mosquito habitats, which affects disease spread patterns. Reliable testing kits such as our wholesale Malaria Pf Test Kit are crucial for adapting to these new challenges by facilitating timely diagnosis in changing environmental conditions.
- The Role of Public-Private Partnerships in Malaria Management
Collaborative efforts between public health sectors and private organizations are vital to achieving widespread access to malaria diagnostics. Initiatives that incorporate our wholesale Malaria Pf Test Kit into broader healthcare strategies illustrate the benefits of such partnerships in combating global health threats.
Image Description